Sequence Group > News

Preparing an EU MDR 2017/745 Submission
- February 16, 2023
- Nick
- EU Regulatory
- No Comments
Preparing an EU MDR Submission The European Union (EU) Medical Device Regulation (MDR) 2017/745 is an important regulation that affects all medical device manufacturers who want to market their products in the EU. Preparing an EU MDR 2017/745 Submission requires a thorough understanding of the requirements outlined in the regulation. This guide will provide an…

The EU MDR 2017/745
- February 16, 2023
- Nick
- EU Regulatory
- No Comments
Table of Contents The European Union Medical Device Regulation (EU MDR) is a major piece of legislation coming into force in Europe. It represents a major overhaul of the existing regulatory framework for medical devices and will have significant implications for manufacturers, healthcare providers, and consumers. The new regulation is intended to enhance patient safety…
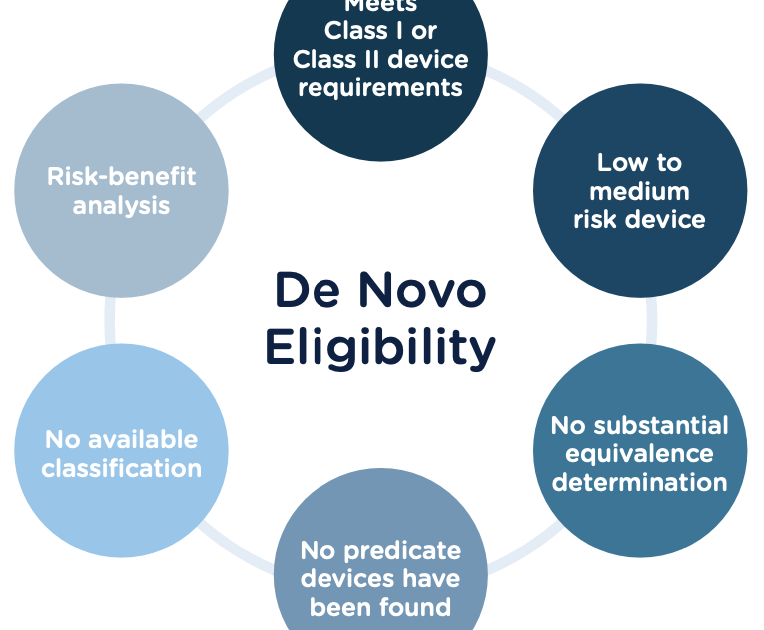
Submitting a 510(k) De Novo to the FDA
- February 16, 2023
- Nick
- US Regulatory
- No Comments
«Sequence Group's regulatory consulting guidance was a tremendous help during the initial growth phase of our organization.»

Larry Johnson
Founder«Sequence Group was vital in providing guidance and support during the design and development process that led the successful depl...

David S. Thomas
Director of R&D«Sequence Group performed the full software validation on our QMS and helped bridge the gap to bring our company to the next level...

