Device Consulting
With a team of highly experienced professionals and a deep understanding of the latest developments in medical device software, AI, and cutting-edge device technology, we are dedicated to helping our clients navigate the complex and ever-changing landscape of regulation and product design.
Our goal is to help our clients bring innovative and effective medical devices to market as efficiently and effectively as possible, through strategic regulatory submissions and cutting-edge design and development solutions. We are committed to delivering exceptional service and results to all of our clients, and we look forward to the opportunity to partner with you on your next project.

Navigating regulatory challenges are at the heart of our approach.
Qualified Specialists
Experienced in all areas of device design and development, from device inception to regulatory submissions.
Individual Approach
Our consultants are experienced in all aspects of the development life cycle and can guide you the entire way.
Great Experience
We work with each client individually to ensure the project goals are met and the objectives are accomplished.
Friendly Customer Service
We take the complexity and headache out of the design process. Contact us today to see what we can do for you.
Our Services
What we do
In the Numbers
20+ years of experience
Projects
Completed Cases
 Consumer Products
Consumer Products
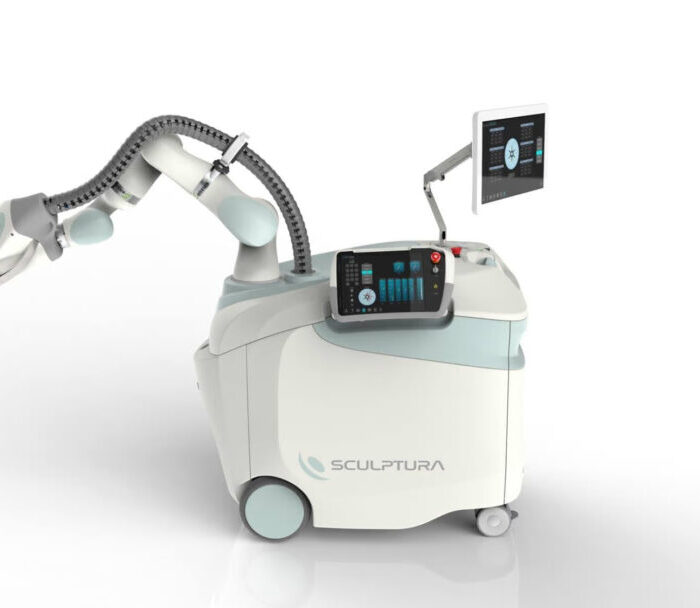
Sculptura IORT Device
Sculptura IORT Device Consumer Products
Consumer Products
Treatment Planning System
Treatment Planning System on iOS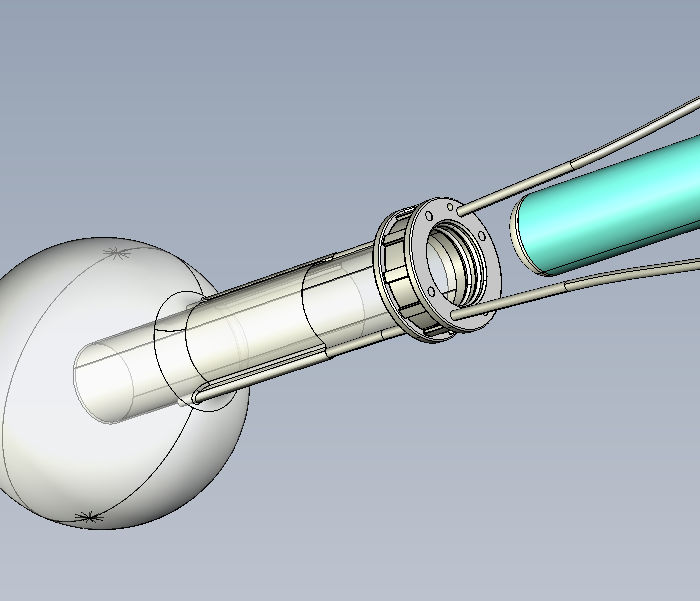 Consumer Products
Consumer Products
TVM Balloon Applicator
TVM Sterile Balloon Applicator Business solutions
Business solutions
Digital QMS Implementation
Successfully launch and validation an organization's digital Quality Management System (QMS) Consumer Products
Consumer Products
New Medical Device Product Development
Oversaw the Design and Development process for company's new flagship device. Compiled entire Design History FileOur Blog
Latest Posts
We provide solutions to the design process
Take the guess work out of the process and consult with us for a proven pathway forward.
-
501 East Las Olas Blvd., Fort Lauderdale, Florida 33301