Table of Contents
-
- A Comprehensive Guide to Submitting an FDA 510k De Novo
- Navigating the FDA 510k De Novo Process: What You Need to Know
- How to Prepare Your Organization for the FDA 510k De Novo Process
- Common Misconceptions About Submitting an FDA 510k De Novo
- Preparing Documentation for an FDA 510k De Novo Submission: What You Need to Know
Submitting an FDA 510k De Novo is a process that is required when a medical device manufacturer seeks to gain approval in the United States for a device that is not substantially equivalent to an already-approved device. The process involves submitting the device to the U.S. Food and Drug Administration (FDA) for review and approval. The 510k De Novo process is a thorough and complex process that requires careful preparation and knowledge of the FDA regulations and guidelines. This article will provide an overview of the 510k De Novo process and what medical device manufacturers need to know when considering submitting an FDA 510k De Novo.
A Comprehensive Guide to Submitting an FDA 510k De Novo
Introduction The FDA 510(k) De Novo classification is an important regulatory step in the medical device industry. It is a process designed to allow a medical device to be classified and commercialized without having to go through the more rigorous and time consuming premarket approval (PMA) process. The FDA De Novo process is an ideal solution for medical device manufacturers who wish to bring new devices to the market quickly and efficiently. This guide will provide a comprehensive overview of the FDA 510(k) De Novo process, including the application requirements, submission process, and review timeline. It will also explain the benefits of the De Novo pathway, as well as the potential risks associated with it. Finally, this guide will provide helpful tips and resources to ensure a successful De Novo submission.
What is a 510(k) De Novo?
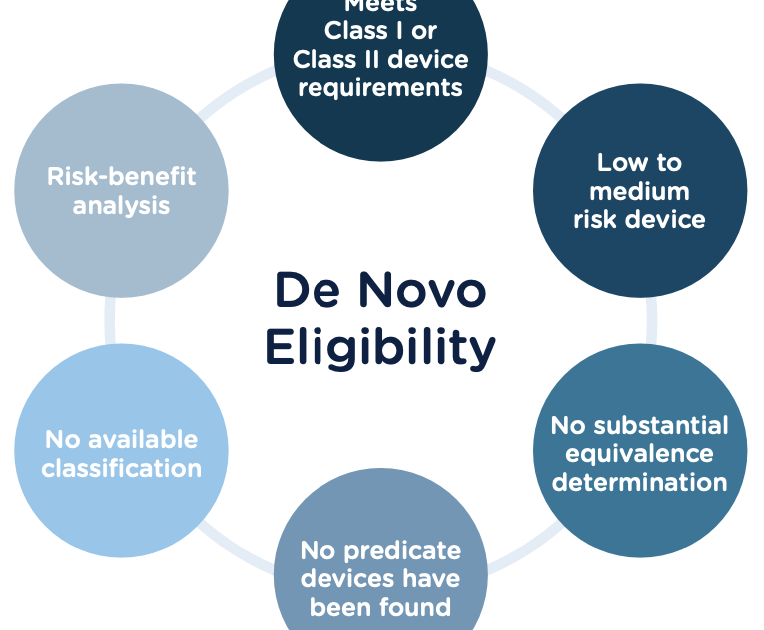
A 510(k) De Novo is a type of medical device premarket notification submitted to the U.S. Food and Drug Administration (FDA). It is used to classify a medical device that does not have an existing classification or a “predicate device” to which it can be compared. The FDA reviews the submission to determine if the device is low to moderate risk and, if so, assigns it a new classification. Benefits of the 510(k) De Novo Process The 510(k) De Novo process offers several advantages for medical device manufacturers.
First, it allows for the rapid introduction of innovative new devices to the market. This can be especially beneficial for companies that are looking to get a jump on their competitors. Secondly, the De Novo process is typically less expensive and time consuming than the PMA process. Finally, the De Novo process allows for the classification of devices without a predicate device, which can provide an additional pathway to market for innovative devices.
Requirements for Submitting a 510(k) De Novo In order to submit a 510(k) De Novo application, the medical device manufacturer must provide the FDA with the following information:
- A description of the device and its intended use;
- A description of the device’s design, components, and materials;
- A description of the device’s performance;
- Supporting scientific evidence and clinical data;
- Labeling and instructions for use;
- Documentation of any premarket or postmarket studies or tests;
- A risk analysis of the device; and
- A summary of the device’s safety and effectiveness. Submission Process The 510(k) De Novo submission process begins with the manufacturer filing an application with the FDA.
Upon receipt of the application, the FDA will review it to determine if the device is low to moderate risk. If so, the FDA will assign the device a new classification and issue a De Novo Order. The manufacturer must then meet the requirements outlined in the Order before the device can be marketed. Review Timeline The FDA typically reviews 510(k) De Novo applications within 90 days of submission. However, the FDA may request additional information or require additional time to review the submission if necessary. Conclusion The FDA 510(k) De Novo process is an important regulatory pathway for medical device manufacturers. It provides a faster and more cost-effective
Navigating the FDA 510k De Novo Process: What You Need to Know
The U.S. Food and Drug Administration (FDA) is responsible for regulating the safety and effectiveness of medical devices. Companies seeking to market a medical device must complete the FDA 510k process, which is a premarket notification that must be submitted to the FDA before they can begin selling the device. For some devices, a traditional 510k submission may be sufficient. However, for certain novel devices, the FDA may require a De Novo 510k submission. Navigating the De Novo 510k process can be a daunting task for companies unfamiliar with the FDA’s regulations and requirements. To help make the process easier, here is what you need to know. First, the De Novo 510k is a request for the FDA to classify a device as low risk. This request is used when the device is novel and does not fit into an existing classification. The De Novo 510k must include comprehensive information about the device, including its purpose, design, and performance data. Second, the FDA will review the request and determine whether the device is low risk or moderate risk. If the device is determined to be low risk, the FDA will assign it a classification and allow it to be marketed. If the device is determined to be moderate risk, the FDA may require additional testing or clinical trials before it can be marketed. Third, the De Novo 510k process is a lengthy one and can take several months to complete. Companies should be prepared to provide the FDA with additional information or data throughout the process. Finally, companies should be aware that the FDA may not approve a De Novo 510k submission. If the request is denied, the company may be able to submit a revised request or may be required to submit a traditional 510k instead. Navigating the FDA 510k De Novo process can be a complicated and time-consuming task. However, by understanding the requirements, staying organized, and working closely with the FDA, companies can ensure that their De Novo 510k submission is successful.
How to Prepare Your Organization for the FDA 510k De Novo Process
The Food and Drug Administration (FDA) 510k De Novo Process is a process in which a medical device manufacturer can receive clearance for a new device based on the safety and effectiveness of another legally marketed device that is not substantially equivalent. The process can provide a quicker path to market for certain low-risk medical devices. Preparing an organization for the FDA 510k De Novo Process can be a challenging endeavor and requires a comprehensive understanding of the steps involved.
- Understand the De Novo Process The De Novo process is a complex regulatory pathway and it is important to understand the process in detail. Review the FDA’s guidance documents and consult with experts in the field to ensure that the device meets the criteria for the De Novo pathway and can be cleared through the process.
- Develop a Comprehensive Submission Plan Once the organization has determined that the device can be cleared through the De Novo process, they must develop a comprehensive submission plan. This plan should include an overview of the device, a risk assessment, clinical data, and other information that is needed to support the submission.
- Prepare the Submission Package The submission package must be prepared in accordance with FDA guidelines. This includes the submission of all required documents, such as a pre-submission request, a summary of safety and effectiveness data, and a scientific rationale for the device.
- Submit the Package to the FDA Once the submission package is complete, it must be submitted to the FDA. The FDA will review the submission and make a determination on whether or not to grant clearance for the device.
- Post-Submission Follow-Up Once the submission has been made, the organization must follow up with the FDA to ensure that the submission is being processed. The FDA may request additional information or clarification on certain aspects of the submission, which must be provided in a timely manner. By following these steps, an organization can ensure that their device is properly prepared for the FDA 510k De Novo Process. It is important to understand the process and to consult with experts who can provide guidance on navigating the process. With the proper preparation, an organization can more easily receive clearance for their device through the De Novo pathway.
Common Misconceptions About Submitting an FDA 510k De Novo
- A De Novo Submission is a Guarantee of FDA Clearance: The De Novo process is designed to evaluate the safety and effectiveness of low to moderate risk medical devices that lack a predicate device. The FDA is not obligated to grant a De Novo request and the decision to grant or deny the request will depend on the results of the review.
- A De Novo Submission is Quick and Easy: A De Novo submission is a complex and lengthy process that requires a thorough understanding of FDA regulations and guidance documents. The submission must include comprehensive information about the device and its intended use, including a detailed design history, product characteristics, pre-clinical and clinical testing, and supporting evidence of the device’s safety and effectiveness. It can take several months to complete a De Novo submission and the FDA may request additional information.
- A De Novo Submission is the Same as 510k Clearance: A De Novo submission is not the same as 510k clearance. A 510k is a premarket notification that is used to demonstrate that a device is substantially equivalent to another legally marketed device. A De Novo is used to evaluate the safety and effectiveness of a new device and is not based on comparison to another legally marketed device.
- A De Novo Submission Automatically Grants Marketing Authorization: A De Novo submission does not automatically grant marketing authorization. The FDA will review the request and may grant or deny the request depending on the results of the review. If the FDA grants the request, a marketing authorization will be issued.
Preparing Documentation for an FDA 510k De Novo Submission: What You Need to Know
The purpose of a 510k de novo submission is to demonstrate to the U.S. Food and Drug Administration (FDA) that a proposed medical device is as safe and effective as a legally marketed device that is already on the market. To do this, the submission must include detailed documentation that meets the FDA’s standards and requirements. The FDA requires the following documents to be included in a 510k de novo submission:
- Pre-Submission Letter: This letter must include the name and address of the manufacturer, a description of the device, the intended use, the classification of the device, and any special labeling requirements.
- Quality System Documentation: The FDA requires manufacturers to demonstrate that they have a quality system in place for device development, production, and marketing. This may include quality system regulations, design control procedures, and post-market surveillance procedures.
- Clinical Protocols: Clinical trial protocols must be provided to demonstrate that the device is effective and safe for its intended use. This may include clinical trial designs, statistical analysis plans, and patient consent forms.
- Device Description: The device description must include a detailed description of the device’s structure, function, and operation. It should also include a list of components used in manufacturing the device and a description of any special labeling requirements.
- Manufacturing Information: The FDA requires manufacturers to provide information about the materials and processes used in manufacturing the device. This may include drawings, specifications, and supplier information.
- Labeling: The FDA requires manufacturers to include a copy of the device’s label in the submission. This should include a description of the device, directions for use, warnings, and any other relevant information.
- Pre-Clinical Testing: Pre-clinical testing results must be included to demonstrate that the device is safe and effective. This may include biocompatibility testing, animal testing, and electrical safety testing.
- Clinical Data: Clinical data must be provided to demonstrate that the device is safe and effective for its intended use. This may include clinical trial results, safety and efficacy data, and patient feedback. By preparing a comprehensive documentation package that meets the FDA’s requirements, manufacturers can ensure that their device will be approved for market. The FDA reviews each submission on a case-by-case basis, so it is important to ensure that all required documentation is included and accurate.
Submitting an FDA 510k De Novo is an important step for companies that wish to introduce a new medical device to the market. This process can be lengthy and complicated, but it is essential to ensuring that the device meets safety and effectiveness standards. Companies should consult with experts to help them understand the process and make sure they are in compliance with all FDA regulations. Ultimately, submitting an FDA 510k De Novo is a necessary step for companies to take in order to ensure their medical device is safe and effective for use.