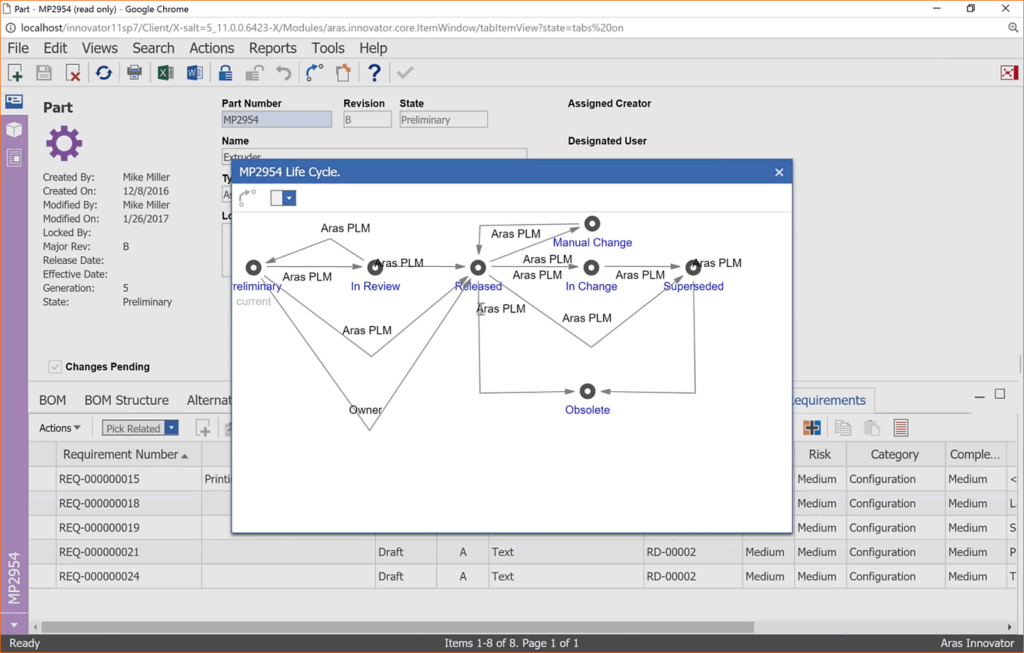
Sequence Group has successfully implemented and validated multiple QMS systems. Most recently, SG deployed a digital PLM system that incorporated all of the product design and development and was expanded to include the company’s entire QMS system. The system was validated for a digital QMS and for 21 CFR Part 11 for digital signatures.
Digital QMS Implementation
Case details
What our customers are saying
Transitioning from a paper system to a digital QMS that also incorporated all device design and quality management system elements, including Design History File, and Device Master Record, to allow for centralized control of the entire device design.
Problems Vs Solutions
No QMS is the same, and no software package works right out the gate. Our team of experts evaluated multiple potential software packages and ultimately deployed a digital QMS that was designed around a PLM system offering central control of a Bill of Materials and all aspects of the QMS.
Sequence Group successfully implemented and deployed the digital QMS that allowed our client to bring on board additional contract manufacturing teams to increase output and productivity. The design successfully passed FDA and EU inspections and saved hundreds of thousands of dollars for the client by allowing for a very streamlined and efficient process flow for the entire QMS.